✔ 100% Authentic Product
👁️ Currently Viewing 2784
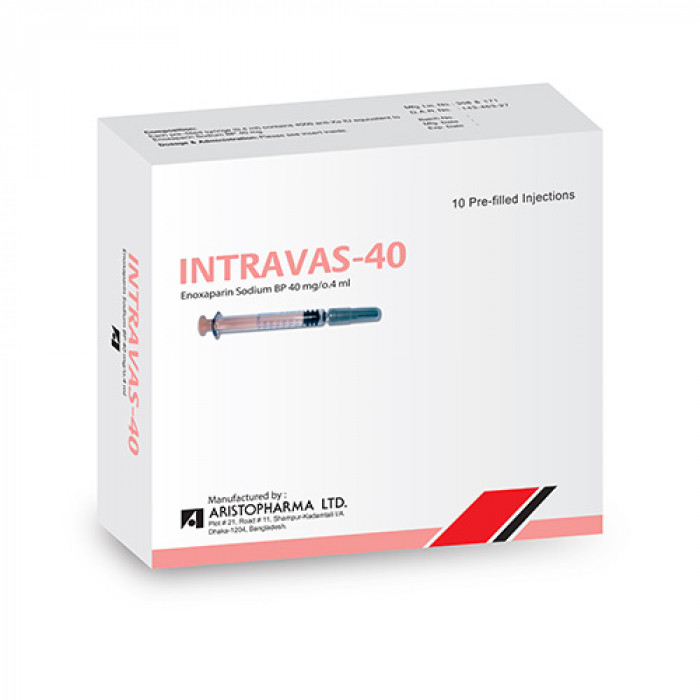
Intravas 40mg Injection
Enoxaparin is used in the treatment and prevention of Blood clots. It prevents the formation of blood clots in the legs, lungs, brain or heart.
Discount
Price: ৳ 380
MRP:
৳
400
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Intravas 40mg Injection 0.4 ml contains Enoxaparin, a low molecular weight heparin (LMWH) that treats harmful blood clots in the blood. It can also prevent blood clots caused by various medical conditions such as unstable angina, post-operation or prolonged bed rest, after a heart attack, and in the tubes of dialysis machines. Intravas 40mg Injection 0.4 ml works by halting the growth of existing blood clots, allowing the body to break them down and preventing harm. It also prevents the formation of new blood clots in the bloodstream.
Safety Advices

Alcohol
UNSAFE
Avoid consumption of alcohol before receiving Intravas 40mg Injection.

Pregnancy
CONSULT YOUR DOCTOR
Intravas 40mg Injection is not recommended for use in pregnancy. Your doctor will weigh the benefits and risks before prescribing this medicine to you.

Breastfeeding
CONSULT YOUR DOCTOR
Intravas 40mg Injection is not recommended for use in breastfeeding. Your doctor will weigh the benefits and risks before prescribing this medicine to you.

Driving
CAUTION
Intravas 40mg Injection does not affect your ability to drive or operate machinery. However, do not drive or handle any heavy tools or machines if your ability is affected by Intravas 40mg Injection.

Kidney
CONSULT YOUR DOCTOR
Intravas 40mg Injection may increase the risk of bleeding if you have kidney problems. Your doctor may adjust the dose of this medicine or prescribe a suitable alternative based on your condition.

Liver
CONSULT YOUR DOCTOR
Intravas 40mg Injection is known to increase the levels of liver enzymes causing serious complications. Therefore, it should be used with caution if you have liver problems.
✔️ Uses of Intravas 40mg Injection
- Treats and prevents blood clots in the body
✔️ How does Intravas 40mg Injection work?
Intravas 40mg Injection works by interfering with the normal clotting process and decreasing the clot-forming ability of the blood.
✔️ Side Effects of Intravas 40mg Injection
- Increases in liver enzymes
- Bruise more easily than usual
- Pink patches on your skin
- Skin rash (hives, urticaria)
- Itchy red skin
- Bruising or pain at the injection site
- Decreased red blood cell count
- High platelet counts in the blood
✔️ Quick Suggestions:
- You have been prescribed Intravas 40mg Injection(0.4ml Each) for the treatment and prevention of blood clots.
- Intravas 40mg Injection (0.4ml Each) increases your risk of bleeding. Be careful while shaving, using sharp objects, or cutting fingernails or toenails.
- Inform your doctor if you are also taking other medicines that increase the bleeding risk like aspirin and NSAIDs.
- Inform your doctor if there is bleeding from gums, nose, or wounds that lasts more than 15 minutes or if blood appears in your urine, stool, or vomit.
- Do not stop taking the medication suddenly without talking to your doctor.
✔️ Indication of Intravas 40mg Injection
Enoxaparin is indicated for:
- Treatment of deep vein thrombosis, with or without pulmonary embolism.
- Treatment of unstable angina and non-Q-wave myocardial infarction, administered concurrently with aspirin.
- Prevention of thrombus formation in the extra-corporal circulation during hemodialysis.
- Prophylaxis of venous thromboembolic disease (prevention of blood clot formation in the veins), in particular those which may be associated with orthopedic or general surgery.
- Prophylaxis of venous thromboembolic disease in medical patients bedridden due to acute illness, including cardiac insufficiency, respiratory failure, severe infections, and rheumatic diseases.
✔️ Pharmacology
Enoxaparin Sodium is a low molecular weight heparin with high anti-Xa activity and low anti-IIa or antithrombin activity. It does not increase bleeding time at the dosages used for various indications. At prophylactic doses, it does not significantly alter activated Partial Thromboplastin Time (aPTT). It does not affect platelet aggregation or the binding of fibrinogen to platelets. Enoxaparin Sodium is primarily metabolized in the liver.
✔️ Dosage & Administration of Intravas 40mg Injection
For the treatment of deep vein thrombosis, with or without pulmonary embolism, the recommended dose of Enoxaparin Sodium is either 100 anti-Xa lU/kg twice daily for 10 days or 150 anti-Xa lU/kg once daily for 10 days. Oral anticoagulant therapy should be initiated as appropriate, and Enoxaparin Sodium treatment should be continued until a therapeutic anticoagulant effect has been achieved.
For the treatment of unstable angina and non-Q-wave myocardial infarction, Enoxaparin Sodium is administered subcutaneously at 100 anti-Xa lU/kg twice daily for 2-8 days, along with oral aspirin (100 to 325 mg once daily). Treatment with Enoxaparin Sodium should be prescribed for a minimum of 2 days and continued until clinical stabilization.
For the prevention of thrombus formation in extracorporeal circulation during hemodialysis, the recommended dose is 100 anti-Xa lU/kg. For patients at a high risk of hemorrhage, the dose should be reduced to 50 anti-Xa lU/kg for double vascular access or 75 anti-Xa lU/kg for single vascular access. Enoxaparin Sodium should be introduced into the arterial line of the circuit at the beginning of the dialysis session.
For prophylaxis of venous thromboembolic disease in surgical patients, the dose varies depending on the type of surgery. For general surgery with a moderate risk of thromboembolism, the recommended dose is 2000 anti-Xa IU (0.2 ml) or 4000 anti-Xa IU (0.4 ml) once daily for 7 to 10 days. For orthopedic surgery with a high risk of thromboembolism, the recommended dose is 4000 anti-Xa IU (0.4 ml) once daily for 7 to 10 days. The first injection should be given before the surgical procedure as per the time specified.
For prophylaxis of venous thromboembolic disease in medical patients, the recommended dose is 4000 anti-Xa IU (0.4 ml) once daily for 6-14 days.
It's important to note that Intravas 40mg Injection should be administered by a doctor or a nurse either intravenously or subcutaneously. In some cases, self-administration may be advised by the physician, and in such cases, the patient should carefully follow the instructions given by the doctor. The appropriate dose and duration of therapy will be determined by your doctor based on your age, body weight, and medical condition.
✔️ Interaction
Intravas 40mg Injection 0.4 ml may interact with several drugs, such as painkillers, blood thinners, drugs used to treat asthma and medications that increase potassium levels in the blood. It is also advised to avoid consuming alcoholic beverages while using this medication.
In addition, Intravas 40mg Injection 0.4 ml may have interactions with various disease conditions, including hemophilia, liver disease, peptic ulcer disease, retinopathy, subacute bacterial endocarditis, active bleeding, hypertension, renal dysfunction, thrombocytopenia, prematurity, and kidney disease. It is important to inform your doctor about any existing medical conditions before using this medication.
✔️ Contraindications
Patients who have previously been allergic to Enoxaparin Sodium, heparin, or other low molecular weight heparins. Active major bleeding and conditions that increase the risk of uncontrolled hemorrhage, such as recent hemorrhagic stroke.
✔️ Pregnancy & Lactation
Enoxaparin Sodium is classified as Pregnancy Category B, indicating that there is no evidence of it crossing the placental barrier in humans. However, since there are no sufficient and well-controlled studies in pregnant women, it should be used during pregnancy only if clearly needed. Pregnant women with mechanical prosthetic heart valves may be at a higher risk for thromboembolism.
It is not known whether Enoxaparin is excreted in human milk. Considering the potential for serious adverse reactions in nursing infants due to the excretion of many drugs in human milk, a decision should be made whether to discontinue nursing or suspend Enoxaparin, taking into account the importance of Enoxaparin to the mother and the known benefits of nursing.
✔️ Precautions & Warnings
Patients should inform their doctor if they are on active treatment with Intravas 40mg Injection 0.4 ml before any surgery is scheduled. If they have any known allergies to Intravas 40mg Injection or any other medicines, they should inform their doctor. For pregnant or breastfeeding individuals, it is important to inform the doctor before using Intravas 40mg Injection 0.4 ml.
Before using Intravas 40mg Injection 0.4 ml, patients should disclose any pre-existing medical conditions, such as stomach ulcer, recent brain/eye/spine surgery, recent bleeding stroke, diabetes, high blood pressure, recent stroke, or kidney/liver disorders. Additionally, it is important to inform the doctor if they are underweight or overweight, or have high levels of potassium in their blood.
✔️ Storage Conditions
- Keep Intravas 40mg Injection) out of reach of children
- Store Intravas 40mg Injection at room temperature
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.